Why EVOOs are not used for HIGH-TEMPERATURE cooking?
According to the popular belief that Extra Virgin Olive Oil (EVOO) must not be used for sauteing, frying, and basically any other stovetop cooking, I feel obliged to tell you that it’s a myth.
Most Culinary Institutes teach that cooking with olive oil lends a “strong flavor” to the food (hiding its natural flavor, “click here“) which may or may not be desirable and so it is best used in salads where you can explore the depth of flavors that this sacred oil has to offer you. I can agree with the second part of the sentence, but regarding the previous one, I would like to present an experiment done at America’s Test Kitchen. A cook heated a good grade of EVOO for 10 minutes at 177ºC then cooled it and a panel of experienced taste experts were presented this oil with refined soybean oil which underwent the same process and the results showed that the taste between the two was indistinguishable (Crosby, 2019). Why so? The volatile compounds (more on the olfactory system here and here) that give olive oil its characteristic flavor (majorly, hexenal and Z-3-hexenal boil at 130ºC and 125ºC respectively) got boiled off leaving behind a bland tasting oil as highly refined vegetable oil. And also, another experiment done at ATK’s kitchen showed that tomato sauce cooked with both EVOO and refined vegetable oil had an indistinguishable taste. So the bottom line is that the food cooked in olive oil doesn’t taste grassy, fruity, and green. Moreover, it might be healthier to use olive oil for cooking because the phenolic antioxidants and anti-inflammatory agents in EVOO survive the cooking process (M. Brenes, 2002).
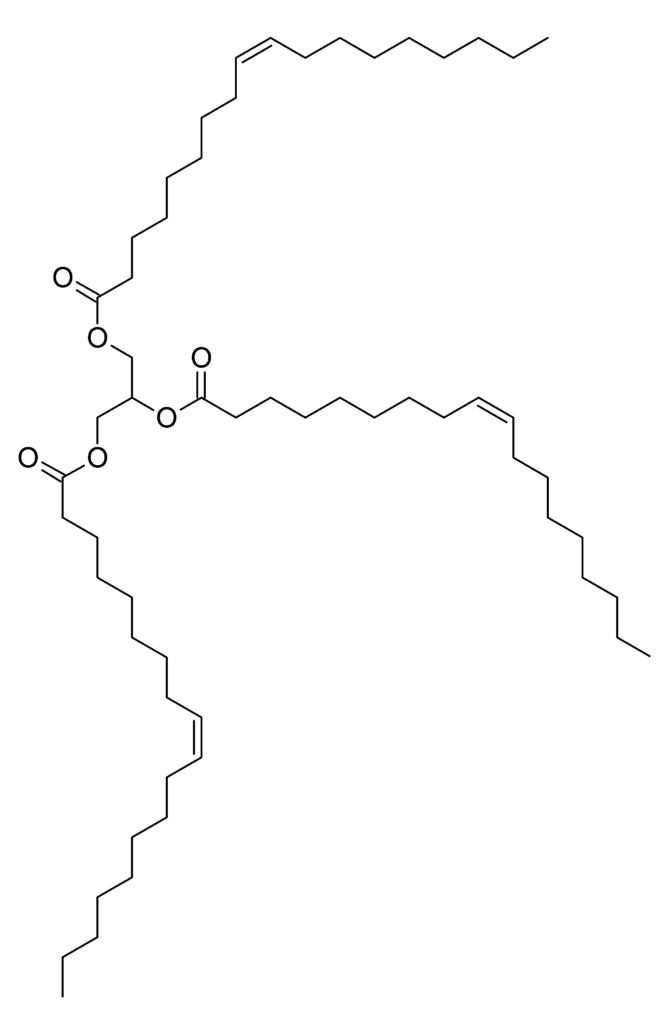
During the cooking process, oil is subject to at least three changes, a) oxidation, b) polymerization , and c) hydrolysis. Oxidation of fatty acids in oils and fats during cooking at high temperatures and in presence of air produces delicious taste and aroma by forming many small volatile compounds such as aldehydes, ketones, and alcohols. But the oxidation at room temperature may result in the formation of compounds that make the oil rancid and unpalatable. The presence of highly monounsaturated fatty acids in olive oil’s glycerides (in the figure) reduces its susceptibility to oxidation as compared to the higher levels of polyunsaturated fatty acids in other vegetable oils. Polymerization happens when you stir fry or deep fry food, you must’ve noticed a ring of sticky yellow stuff while frying, that’s just oxidized and polymerized oil and its volume depends on how long the oil was heated. And finally, hydrolysis is the breakdown of triglycerides in the presence of food specks and water while cooking producing toxic and undesirable compounds, thus, it is recommendable to change the oil in the fryer frequently because there’s nothing more disappointing than being served fried foods with off flavors and colors. Also, people consider olive oil to have a lower smoking point and hence exclude it from the shelf of cooking oil, which is just a tale rather than a fact. But it depends more on the grade of refinement of the oil. A smoking point is reached when the triglycerides are broken down to glycerol and free fatty acid molecules, and the glycerol is rapidly dehydrated to the toxic acrolein (Roman Papoušek, 2014), which is the resultant blue gaze seen over the smoking oils.
The smoking point of unfiltered EVOO can be as low as 191ºC whereas that of a high-quality cold-pressed one is about 207ºC and that of a highly refined olive oil is about 242ºC (Crosby, 2019). I believe we don’t really need to go to the smoking point of oil for cooking because that is when it starts to break down and the margin of error (taste-wise) reduces to zero. The only sensible reason for not using olive oil in high-temperature cooking is its price. Because dumping half a liter of olive oil to fry some chicken, instead of cheaper vegetable oil, seems like a waste. And we’re not made of “Plata”. But still, we can buy good quality and cheaper EVOO.
References:
Alfred, T., Matthäus, B., & Fiebig, H. J. (2002). Fats and fatty oils. Ullmann’s Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH.