What is coagulation and how does it work?
We all are aware that cheese is obtained from coagulating milk, either through the action of acid or enzymes. But to understand the concept of “splitting” of milk we need to know what is milk and what constitutes milk.
Milk is a nutrient-rich liquid produced by the mammary glands of mammals. Milk mostly consists of fat, protein, lactose (a kind of sugar), and water. The milk fat is suspended in the water as fine droplets, which makes it an emulsion (oil-in-water). Milk also contains a lot of proteins that, in this case, are mostly whey (lactoglobulin) and casein (κ-caseins). These casein proteins are poorly soluble in water and build spherical structures called micelles (where did we last see micelles?) that allow them to stay in suspension as if they were soluble. There are a lot of theories defining the structure of these micelles but they all share one thing in one and that is the outermost layer consists of strands of one type of protein, κ-caseins. These kappa-casein molecules all have a negative electrical charge and therefore repel each other forming the abovementioned micelles.
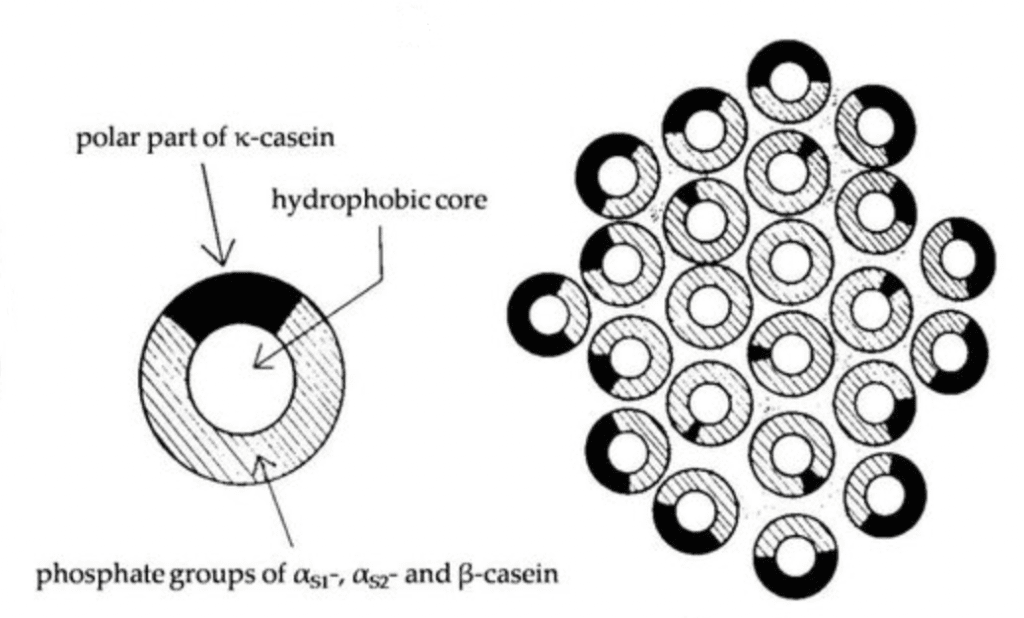
Dumpler (2017)
The other proteins that stay in the whey water even after the curdling (getting there!) that stay in the liquid that is left behind are collectively known as whey proteins. β-lactoglobulin is the most common whey protein.
So what does it mean when someone says “the milk is split or coagulated?”. Simply, coagulation means when the liquid milk is transformed in some way to get a solid mass (which is used to make cheese or other products) leaving behind the whey. The micelle structures can easily be disrupted or changed, and once altered they cannot be reformed by the action of acids, enzymes, or even sometimes heat.
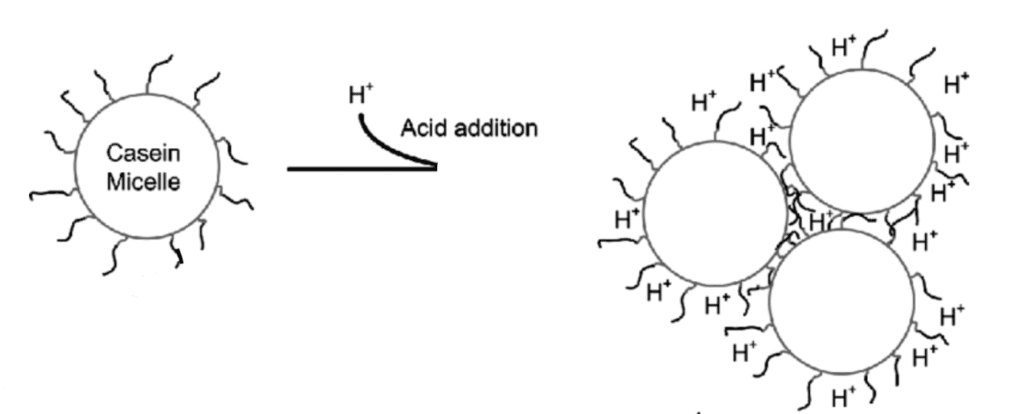
Coagulation by Acid (Lowering the pH)
Adding acid to something is like adding a positive charge, let’s recall the Brønsted–Lowry definition of an acid which is “an acid is a molecule or ion capable of either donating a proton”. The most commonly used acid in the kitchen is acetic acid (or vinegar) or lemon juice. So when we add acid or protons (H+) to milk that has casein micelles, which are negatively charged a neutralization reaction takes place and the micelles lose their negative charge which allows them to bump and stick and this effect is the most prominent at the isoelectric point of casein, pH = 4.6.
Coagulation by Enzymes
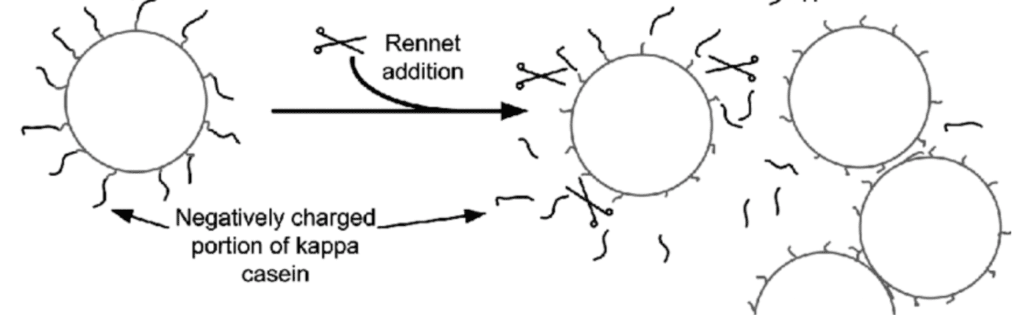
As discussed earlier, κ-caseins strands are present at the outermost layer of the micelles, which are like negatively charged hairs that inhibit the clotting of the proteins and keep the milk homogenized. And the most common ingredient to curdle the milk used in the cheese-making industry is rennet. It is a set of enzymes (there are also some vegetarian sources) that is naturally present in the stomach of ruminants and contains chymosin, pepsin, and lipase.
The main player in this cocktail of enzymes is chymosin, which acts as scissors cutting off the “κ-caseins hair” and thus neutralizing the micelles and the say thing happens they lose their charge, bump into each other and stick.
PS: One of the main differences in the curds formed by acids and enzymes is that the former is much softer that is because the acid also dissolves the “calcium glue” in the intra-micellar structure (more on this here). And that is also a reason why different cheese has different textures (there are a lot of reasons!!).
References
- Dumpler, Joseph. (2017). On the heat stability of concentrated milk systems – Kinetics of heat-induced dissociation and aggregation of casein micelles.